FarmaKology Newsletter - Issue #33
How do we identify generic entrants before they get FDA approval?
DrugPatentWatch can help identify generic entrants at the earliest stages
Many clients come to DrugPatentWatch seeking to identify generic entrants well before they launch.
CDMOs, equipment manufacturers, regulatory consultants, and API manufacturers all have a desire to identify future generic manufacturers at the earliest possible opportunity — well before their generic drug applications are approved. The challenge in identifying generic entrants at the early stage is that the FDA does not disclose the names of generic ANDA applicants until they have granted approval for their drug applications.
This customer success story describes some of the methods DrugPatentWatch clients use to identify companies planning generic drug launches.
The easy way to identify near-term generic launches
Before diving into identifying generic entrants at the earliest possible stages, it is worth noting a quick tactic to identify impending launches. While the FDA does not disclose the name of companies which have submitted drug applications until they approve those applications, looking at tentative approvals can reveal the names of generic companies who are waiting for key patents or regulatory approvals to clear. Once these key regulatory protections and patents have expired these generics are cleared to launch.
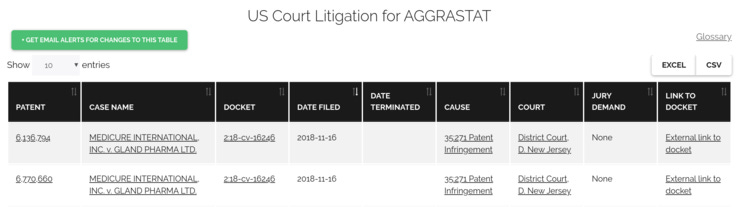
Follow litigation to see who’s challenging drug patents
The first generic entrants will often challenge the validity of drug patents to secure the earliest possible launch date. While the FDA does not disclose the names of these patent-challenging generic firms, they are required to inform the branded drug maker of their patent challenge. The branded firm must then initiate litigation to prevent the generic from launching.
So, looking at records of drug patent litigation can identify potential generic entrants.
These companies have invested in formally filing for generic drug approval, and they have exposed themselves to litigation, so their intent to launch a generic drug is clear. Often these companies are at the earliest stages of development and represent strong opportunities for CDMOs, API suppliers, or lab equipment manufacturers.
What about 505(b)(2) hybrid drugs?
It may also be possible for generic to launch by the 505(b)(2) hybrid route to either develop an improved version of a drug, or to work around patents.
Looking at litigation or tentative approvals is unlikely to identify companies working around patents or developing improved versions of drugs, but there is another place to look: clinical trials.
By looking at clinical trials for names of common 505(b)(2) companies, or looking at the trials themselves for studies focused on alternate formulations (e.g. extended release versions of existing drugs) can identify companies likely to submit New Drug Applications for these improvements.
A benefit of looking at clinical trials is that it can identify companies well before they submit drug approval applications, making them great targets for business development for regulatory consultants and attorneys, and for patent litigation firms.